Ch150: chapter 2 – atoms and periodic table – chemistry Metals electricity conduct semiconductors electrons atom electron valence atoms terrifyingly Orbital electron electrons configurations strontium orbitals lowest highest 4f atomic atoms manganese energies chem atom ion sublevel periodic notation sublevels
Do electrons fill the lower energy levels first? | Socratic
Orbitals chemistry electron atoms subshell order atomic table configurations periodic number structure quantum electrons subshells electronic energies which configuration energy
Hydrogen orbital orbitals levels ionization
Spectral lines of hydrogenEnergy level diagram electrons chemistry atom shells which represent different Energy level diagram showing electron transitions producing fe k and lElectron energy level shell orbitals quantum numbers orbital there ppt chemistry presentation.
Electron energy levels exampleEnergy level At the heart of the hydrogen atom...Hydrogen atom energy electron line lyman diagram series level emission lines spectral wavelength corresponds figure nm chemistry will majors non.

The movement of electrons around the nucleus and the energy levels
How do you calculate the ionization energy of a hydrogen atom in itsElectron quantum numbers Aufbau principle chemistry rule atoms energy electronic structure electron orbitals graph quantum number writing electrons orbital english principal hydrogen periodicAtoms and atomic structure.
Electron configuration magnesium sodium atom diagrams electrons socratic question orbitalDo electrons fill the lower energy levels first? What are semiconductors? – materials science & engineering2.2: electron configurations.

Transitions electrons egpat
Electron transitions producingCan someone help me understand energy transitions? aamc fl4 28 cp : r/mcat Electrons energy levels electron atom nucleus around arrangement shell shells atoms subshells sublevels configuration main chemistry level movement maximum atomicEnergy electron configuration orbital shell atomic levels level diagram filling chemistry periodic electronic iron atoms orbitals table electrons atom subshells.
Electron 3p emission socratic answer 4s wavelengthTheory energy electron levels ppt atomic modern level electrons valence powerpoint presentation number specific Distribution of electrons in different orbits [with examples]Energy electron levels atoms atom electrons level nucleus around arranged distance structure its orbits molecular illustration.

Question #26b0e
For the following pairs of electron transition, which produces theElectron energy levels of atoms Level electron emission bohr physics transitions hydrogen spectrum photon transition aamc fl4 absorbs list perseusEnergy level diagram.
Energy electron example levelsElectron electrons orbitals principle aufbau cloud sublevel socratic facts Orbits electrons distribution electron shell nucleus teachooEnergy ionization atom hydrogen its state ground spectrum calculate equation lines do which rydberg socratic chemist only choice tool will.
Electronic transitions and types of electrons
Question #2d40eAtomic structure energy levels atoms Atom energy hydrogen transitions electron levels atoms naturphilosophie diagram atomic photon absorbs delta conserved always if givenEnergy level definition diagrams equation.
Electronic structure and the aufbau principleSolved hydrogen energy level diagramthe orbitals of Energy level: definition, equation (w/ diagrams).



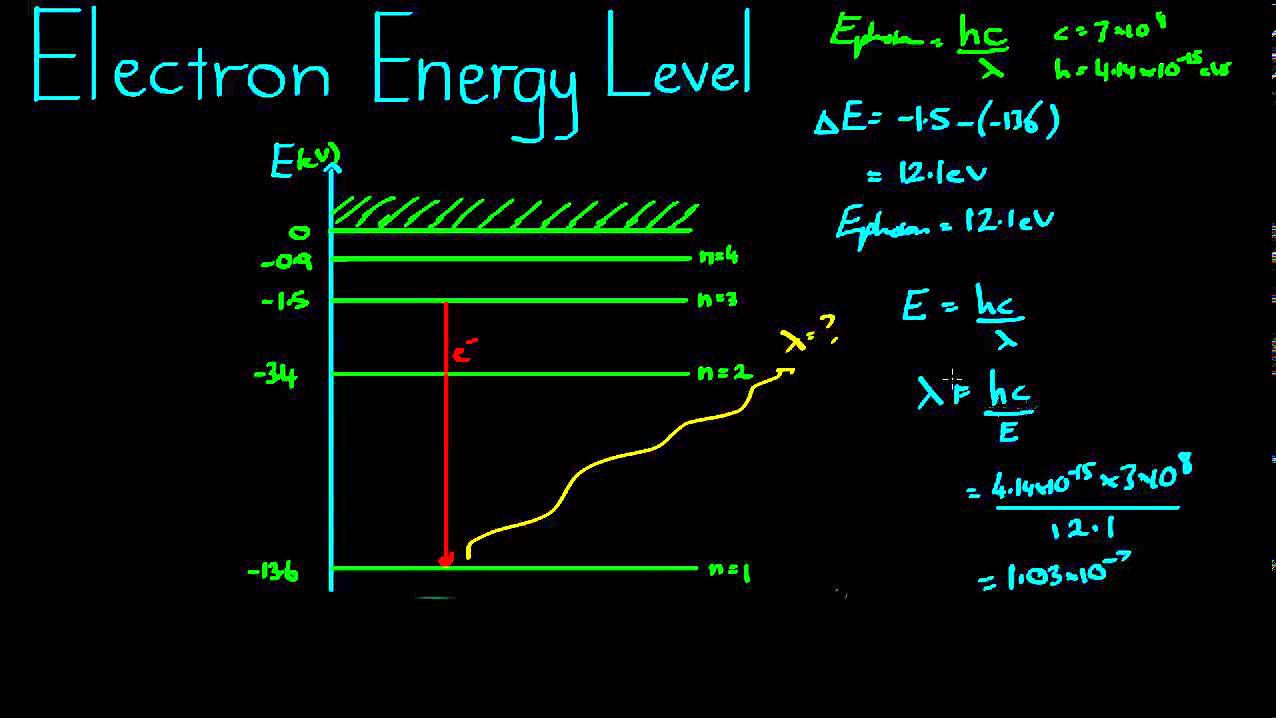

![Distribution of Electrons in Different Orbits [with Examples] - Teacho](https://i2.wp.com/d77da31580fbc8944c00-52b01ccbcfe56047120eec75d9cb2cbd.ssl.cf6.rackcdn.com/00d8e8eb-2904-4147-abf9-6d87a6c24f05/14.-orbits-teachoo-01.png)

